白藜芦醇提取物
白藜芦醇作为一种强效的抗氧化剂在外用的护肤品中的利用价值,是业界中公认的强大。它是由日本人在1940年首次从毛叶藜芦(Veratrum grandiflorum)的根部分离获得,是一种多酚类化合物,广泛存在于葡萄,虎杖,桑葚,花生等植物中。
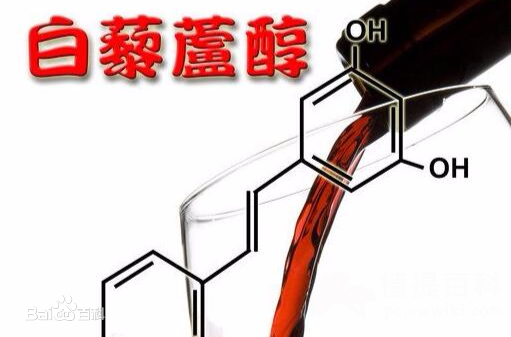
目前外用白藜芦醇国内外还没有浓度使用限制,天然等同来源的白藜芦醇反式结构纯度极高,安全性好。
白藜芦醇详细介绍
【中文品名】 (bái lí lú chún)
【药效类别】抗肿瘤药,抗血栓药
【通用药名】RESVERATROL
【别 名】Protykin Resveratrol (Natrol), Resveratrol Antioxidant Protection (Source Naturals)
【化学名称】 1,3-Benzenediol, 5-[(1E)-2-(4-hydroxyphenyl)ethenyl]
【中文化学名称】3,4′,5-三羟基-反-均二苯代乙烯(3,4′,5-trihydroxysitlbene)
【CA登记号】[501-36-0]
【结 构 式】

RESVERATROL
【分 子 式】CHNOPS·
【分 子 量】228.24
【来源】葡萄皮葡萄籽、白藜芦、日本虎杖、金银花、蓝莓、蔓越莓、花生皮、桑白皮、菠萝蜜、大黄漏芦、欧洲赤松、云杉、紫荆花、桉树
【理化性质】为无色无味针状结晶,难溶于水(0.03 g/L),易溶于乙醚、氯仿、甲醇、乙醇(50 g/L)和丙醇,在波长365nm的紫外光照射下能产生荧光,并能和三氯化铁-铁氢化钾起显色反应。
【存在形式】顺式白藜芦醇、反式白藜芦醇、顺式白藜芦醇苷和反式白藜芦醇苷四种
【收录药典】各国药典
【首次发现】1940年首次从毛叶藜芦(veratrum grandiflorum)的根部分离得到
【性 状】为无色无味针状结晶
【药理作用】
(1)抗肿瘤作用
(2)治疗心血管病作用
(3)抗氧化、抗自由基作用
(4)保肝作用
(5)雌激素样作用和影响骨代谢作用
(6)调节免疫
(7)抗病毒
(8)抗细菌及真菌
(9)抗变态反应
(10)辐射防护
(11)预防急性传染性非典型肺炎
【用 途】广泛应用于医药、保健品、化妆品和食品添加剂等领域
【推荐合成路线】

白藜芦醇的合成
一、3,5-二甲氧基苄醇(2)的合成
3,5-二甲氧基苯甲酸(5.0g,27.5mmol)加至无水乙醚(150 ml)中,搅拌下分批加入LiAlH4(1.0g,26.3mmol),微回流反应18h。缓慢滴加稀盐酸至无气泡产生且未分层时停止,得灰色混悬液。过滤,滤液减压浓缩回收乙醚,残余物静置后凝固成白色固体(2)(4.3g,93.2%),mp 46~47℃。
二、3,5-二甲氧基溴苄(3)的制备
(2)(1.g,11.3mmol)加至甲苯(10 ml)中,搅拌下加入40%氢溴酸(5ml),于60~80℃油浴中反应3h后,减压浓缩回收甲苯。滤出析出的固体,水洗至中性,甲醇重结晶,得棕色片状晶体(3)(1.58g,56.6%),mp 71~72℃。
三、3,5,4′-三甲氧基二苯乙烯(5)的制备
(3)(2.2g,9.7 mmol)和亚磷酸三乙酯(5ml)于搅拌下回流反应1h,减压蒸除过量的亚磷酸三乙酯,得淡黄色液体磷酸3,5-二甲氧基苄基二乙酯
(4),直接用于下一步反应。
所得(4)溶于DMF(7ml),于冰水浴中加入甲醇钠(1.2g,22.2mmol)并搅拌15min。向上述反应液中加入茴香醛(2.3g,16.9mmol),冰水浴中反应1h,室温反应过夜。所得黄色混悬液倾至冷水(60 ml)中,过滤,滤饼用水洗涤,再分别用95%乙醇、无水乙醇二次重结晶,得白色片状晶体(5)(1.92g,73%),mp 56.5℃。元素分析(C17H18O3)实测值(计算值,%):C 75.82(75.53),H 6.80(6.71)。
四、白藜芦醇(1)的合成
(5)(0.27g,1mmol)加至二氯甲烷(5ml)中,搅拌至溶解。滴加1mol/L的BBr3的二氯甲烷溶液(10ml),室温搅拌反应2h,TLC跟踪反应[展开剂为丙酮—正己烷(1:2)]。反应液倾至冰水(30ml)中,用乙酸乙酯萃取(20ml×2),合并有机层,用无水硫酸镁干燥,过滤后减压蒸除溶剂,得淡黄色固体。用50%乙醇重结晶,得白色结晶(1)(0.20g,90%),mp 256~257℃。
【光谱数据】
1HNMR(DSMO-d6):δ6.11(1H, t, H-4),6.38(2H, d, H-2,6), 6.75(2H, d, H-3′,5′),6.81, 6.93(each 1H, d, -CH=CH-),7.39(2H, d, H-2′,6′),9.18(2H, s, OH-3,5),9.54(1H, s, OH-4′)
【其它合成路线】
Wittig反应
通过Wittig试剂(磷Ylide等)与醛、酮的羰基发生亲核加成反应,形成烯烃。此方法简便,但产率较低。目前,已有很多利用Wittig反应合成白藜芦醇的报道。
【白藜芦醇的生物合成】
白藜芦醇作为芪类次生代谢物广泛存在于种子植物中,其主要通过苯丙氨酸代谢途径合成的;其中最为关键的调节酶是二苯乙烯合酶,也叫做称芪合酶(Stilbenesynthase,简称STS)。

植物芪类次生代谢物生物合成途径
芪合酶在植物中多以基因家族存在,到目前为止,已克隆的芪合酶可分为2种类型:一种为白藜芦醇合成酶(Resveratrolsynthase,简称RS)(EC2.3.1.95),又被称为3,4′,5-三羟基二苯乙烯合酶,主要存在与花生以及葡萄中,以丙二酰辅酶A和4-香豆酰辅酶A为底物合成白藜芦醇;第二种为赤松素合成酶(EC2.3.1.146),以丙二酰辅酶A和肉桂酰辅酶A为底物合成赤松素(Pinosylvin)。研究表明,白藜芦醇合成酶是白藜芦醇合成代谢途径中最后一个起作用的关键酶,也是整个合成途径中唯一必需的合成酶。
【参考文献】
[1] Resveratrol: A Review https://en.wikipedia.org/wiki/Resveratrol
[2] Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000; 141:3657-3667.
[3] Burkitt MJ, Duncan J. Effects of trans-resveratrol on copper-dependent hydroxyl-radical formation and DNA damage: Evidence for hydroxyl-radical scavenging and a novel. Glutathione-sparing mechanism of action. Arch Biochem Biophys. 2000; 381:253-263.
[4] Cao G, Prior RL. Red wine in moderation: Potential health benefits independent of alcohol. Nutr Clin Care. 2000; 3:76-82.
[5] Chun YJ, Kim MY, Guengerich FP. Resveratrol is a selective human cytochrome P450 1A1 inhibitor. Biochem Biophys Res Commun. 1999; 262:20-24.
[6] Cichewicz RH, Kouzi SA, Hamann MT. Dimerization of resveratrol by the grapevine pathogen. Botrytis cinerea. J Natl Prod. 2000; 63:29-33.
[7] Ciolino HP, Yeh GC. Inhibition of aryl hydrocarbon-induced cytochrome P450 1A1 enzyme activity and CYP1A1 expression by resveratrol. Mol Pharmacol. 1999; 56:760-767.
[8] Doherty JJ, Fu MM, Stiffer BS, et al. Resveratrol inhibition of herpes simplex virus replication. Antiviral Res. 1999; 43:145-155.
[9] Dubash BD, Zheng BL, Kim CH, et al. Inhibitory effect of resveratrol and related compounds on the macromolecular synthesis in HL-60 cells and the metabolism of 7,12-dimethylbenz[a]anthracene by mouse liver microsomes. In: Shahidi F, Ho C-T, eds. Phytochemicals and Phytopharmaceuticals. Champaign, IL: AOCS Press; 2000:314-320.
[10] Fontecave M, Lepoivre M, Elleingand E, et al. Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 1998; 421:277-279.
[11] Frémont L. Biological effects of resveratrol. Life Sci. 2000; 66:663-673.
[12] Frémont L, Belguendouz L, Delpal S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci. 1999; 64:2511-2521.
[13] Gehm BD, McAndrews JM, Chien P-Y, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci USA. 1997; 94:14138-14143.
[14] Holmes-McNary M, Baldwin AS Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000; 60:3477-3483.
[15] Hsieh TC, Juan G, Darzynkiewicz Z, Wu JM. Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21 (WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2. Cancer Res. 1999; 59:2596-2601.
[16] Hung L-M, Chen J-K, Huang S-S, et al. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovascular Res. 2000; 47:549-555.
[17] Jang M, Cai L, Udeani GO, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997; 275:218-220.
[18] Jang M, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Drugs Exp Clin Res. 1999; 25:65-77.
[19] Kirk RI, Deitch JA, Wu JM, Lerea KM. Resveratrol decreases early signaling events in washed platelets but has little effect on platelet aggregation in whole blood. Blood Cells Mol Dis. 2000; 26:144-150.
[20] Martinez J, Moreno JJ. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem Pharmacol. 2000; 59:865-870.
[21] Nielsen M, Ruch RJ, Vang O. Resveratrol reverses tumor-promoter-induced inhibition of gap-junctional intercellular communication. Biochem Biophys Res Commun. 2000; 275:804-809.
[22] Pace-Asciak CR, Hahn S, Diamandis EP, et al. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995; 235:207-219.
[23] Paul B, Masih I, Deopujari J, Charpentier C. Occurrence of resveratrol and pterostilbene in age-old darakchasava, an ayurvedic medicine from India. J Ethnopharmacol. 1999; 68:71-76.
[24] Pinto MC, García-Barrado JA, Macías P. Resveratrol is a potent inhibitor of the dioxygenase activity of lipoxygenase. J Agric Food Chem. 1999; 47:4842-4846.
[25] Ray PS, Maulik G, Cordis GA, et al. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Rad Biol Med. 1999; 27:160-169.
[26] Sanders TH, McMichael RW Jr, Hendrix KW. Occurrence of resveratrol in edible peanuts. J Agric Food Chem. 2000; 48:1243-1246.
[27] Schneider Y, Vincent F, Duranton B, et al. Anti-proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 2000; 158:85-91.
[28] Soleas GJ, Diamandis EP, Goldberg DM. Resveratrol: A molecule whose time has come? And gone? Clin Biochem. 1997; 30:91-113.
[29] Stewart JR, Christman KL, O’Brian CA. Effects of resveratrol on the autophosphorylation of phorbol ester-responsive protein kinases. Biochem Pharmacol. 2000; 60:1355-1359.
[30] Subbaramaiah K, Chung WJ, Michaluart P, et al. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J Biol Chem. 1998; 273:21875-21882.
[31] Subbaramaiah K, Michaluart P, Chung WJ, et al. Resveratrol inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Ann NY Acad Sci. 2000; 889:214-223.
[32] Tang W, Eisenbrand G. Chinese Drugs of Plant Origin. Berlin: Springer-Verlag; 1992; 787-791.
[33] Tessitore L, Davit A, Sarotto I, Caderni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21CIP expression. Carcinogenesis. 2000; 21:1619-1622.
[34] Tomera JF. Current knowledge of the health benefits and disadvantages of wine consumption. Trends Food Sci Technol. 1999; 10:129-138.
[35] Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999; 126:673-680.
[36] Zou J, Huang Y, Chen Q, et al. Suppression of mitogenesis and regulation of cell cycle traverse by resveratrol in cultured smooth muscle cells. Int J Oncol. 1999; 15:647-651.
[37] 中国医药工业杂志,2003, 34(9), 428
[38] 中国药物化学杂志,2002,12(3):152
[39] Can.J.Chem., 1970,48:1554
[40] 中国医药工业杂志,2000,31(7):334
标签:抗氧化剂
声明:以上信息没有经过国家食品药品监督管理局评估和确认,来自互联网公开发表的文献,仅供参考。


